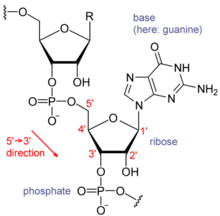
Each nucleotide in RNA contains a ribose sugar, with carbons numbered 1 through 5. A base is attached to the 1 position, generally adenine (A), cytosine (C), guanine (G) or uracil (U). Adenine and guanine are purines, cytosine and uracil are pyrimidines. A phosphate group is attached to the 3 position of one ribose and the 5 position of the next. The phosphate groups have a negative charge each at physiological pH, making RNA a charged molecule (polyanion). The bases may form hydrogen bonds between cytosine and guanine, between adenine and uracil and between guanine and uracil. However other interactions are possible, such as a group of adenine bases binding to each other in a bulge, or the GNRA tetraloop that has a guanine–adenine base-pair.
An important structural feature of RNA that distinguishes it from DNA is the presence of a hydroxyl group at the 2 position of the ribose sugar. The presence of this functional group causes the helix to adopt the A-form geometry rather than the B-form most commonly observed in DNA.This results in a very deep and narrow major groove and a shallow and wide minor groove. A second consequence of the presence of the 2-hydroxyl group is that in conformationally flexible regions of an RNA molecule (that is, not involved in formation of a double helix), it can chemically attack the adjacent phosphodiester bond to cleave the backbone